Report Virlaza™ in the Context of Lung Inflammation and Fibrosis in COVID-19
Responsible:
PROF. DR. RODOLFO DE PAULA VIEIRA
Laboratory of Pulmonary Immunology and Exercise Immunology (LABPEI) of the Federal University of São Paulo (UNIFESP), Rua Talim 330, room 119, São José dos Campos, Brazil, 12231-280.
The experiments were done using two cells which have a central role in the physiopathology of COVID-19, BEAS-2B cells (bronchial epithelial cells) and MRC-5 cells (lung fibroblast cells), which were stimulated by whole blood cells from COVID-19 patient with viral load ranging CT 15-18, obtained from acute phase of disease (maximum of 24 hours of hospitalization). In summary, 5x104 BEAS-2B cells and 1x104 MRC-5 cells were cultivated in 2 ml of freshwhole blood of COVID- 19 patient, for an initial incubation with 50ul of VIRLAZA for 1 hour, followed by an additional period of incubation of 3 hours, into a CO2 incubator, with 5%CO2 and 37ºC degrees. After 4 hours of incubation, the cells were harvested and centrifuged at 900g at 4º degrees, for 7 minutes. The supernatant was used for measurement of pro-inflammatory cytokines (IL-1beta, IL-6, IL-8 and TNF-alpha), which are key cytokines for COVID-19 physiopathology, progression and severity, as well as of anti-inflammatory cytokines (IL-1RA and IL-10).
The results demonstrated that VIRLAZA significantly inhibited the activation of bronchial epithelial cells (BEAS-2B), as it happens in the real-life in vivo context during COVID-19 infection, since VIRLAZA inhibited the synthesis andrelease of IL-1beta (p<0.001), IL-6 (p<0.05), IL-8 (p<0.05) and TNF-alpha (p<0.001). Of note, according to theprotocol used, adding VIRLAZA only after 1 hour incubation of BEAS-2B cells with infected blood from COVID-19 patient, strongly suggest that VIRLAZA could be successfully used in the early phase for COVID-19 patients, to avoid the aggravation of the disease, which happens due to intense synthesis and release of pro- inflammatory cytokines by bronchial epithelial cells. In terms of possible immunological mechanism underlying such effects, the present study revealed that VIRLAZA induced synthesis and release of IL-1RA in BEAS-2B cells, which is a potent endogenous anti- inflammatory cytokine. In addition, following this initial pro-inflammatory response, an activation of lung fibroblasts is seemed in COVID-19 patients, which leads to lung fibrosis, compromise the lung function, the quality of life and the general health status of such patients for a long period. In this case, the present study demonstrated that VIRLAZA efficiently reduced lung fibroblasts (MRC-5 cells) activation, since VIRLAZA reduced the synthesis and release of IL-1beta (p<0.001), IL-6 (p<0.001) and TNF-alpha (p<0.001), which are classical cytokines used as biomarker of fibroblast activation. Importantly, the levels of IL-8 were not changed in MRC-5 after stimulation with infected blood of COVID-19 patients and also not changed after VIRLAZA stimulation. In this sense, is plausible to hypothesize that VIRLAZA, also could inhibit the lung fibrosis observed in more severe cases of COVID-19. Again, concerning the possible anti-inflammatory immunological mechanism involved in the effects ofVIRLAZA, its was able to induce the synthesis and release of the anti-inflammatory cytokine IL-1RA, which is the trigger anti-inflammatory cytokine suggesting deactivation of lung fibroblasts, induced by COVID-19. So, with theseconsistent results, we are able to prove a potent anti-inflammatory and anti-fibrotic effect of VIRLAZA in the context ofCOVID-19, open a new opportunity for early intervention in individuals with COVID-19. Thus, considering such results, a clinical trial using VIRLAZA for COVID-19 patients from different severities are urgently required.
The graphs presenting all the results are displayed bellow.
***p<0.001; **p<0.01 and *p<0.05. Co = control group (only the cells);
VR = VIRLAZA group (cells + VIRLAZA);
SarsCov2 = Infected group (cells incubated with blood of patient infected with COVID-19) and SarsCov2 + VR = Infected group treated with VIRLAZA.
We clarify that the levels of IL-10 (an immunomodulatory and sometimes anti-inflammatory cytokine) were also measured, but no differences among all groups were found.
PROF. DR. RODOLFO DE PAULA VIEIRA
Laboratory of Pulmonary Immunology and Exercise Immunology (LABPEI) of the Federal University of São Paulo (UNIFESP), Rua Talim 330, room 119, São José dos Campos, Brazil, 12231-280.
The experiments were done using two cells which have a central role in the physiopathology of COVID-19, BEAS-2B cells (bronchial epithelial cells) and MRC-5 cells (lung fibroblast cells), which were stimulated by whole blood cells from COVID-19 patient with viral load ranging CT 15-18, obtained from acute phase of disease (maximum of 24 hours of hospitalization). In summary, 5x104 BEAS-2B cells and 1x104 MRC-5 cells were cultivated in 2 ml of freshwhole blood of COVID- 19 patient, for an initial incubation with 50ul of VIRLAZA for 1 hour, followed by an additional period of incubation of 3 hours, into a CO2 incubator, with 5%CO2 and 37ºC degrees. After 4 hours of incubation, the cells were harvested and centrifuged at 900g at 4º degrees, for 7 minutes. The supernatant was used for measurement of pro-inflammatory cytokines (IL-1beta, IL-6, IL-8 and TNF-alpha), which are key cytokines for COVID-19 physiopathology, progression and severity, as well as of anti-inflammatory cytokines (IL-1RA and IL-10).
The results demonstrated that VIRLAZA significantly inhibited the activation of bronchial epithelial cells (BEAS-2B), as it happens in the real-life in vivo context during COVID-19 infection, since VIRLAZA inhibited the synthesis andrelease of IL-1beta (p<0.001), IL-6 (p<0.05), IL-8 (p<0.05) and TNF-alpha (p<0.001). Of note, according to theprotocol used, adding VIRLAZA only after 1 hour incubation of BEAS-2B cells with infected blood from COVID-19 patient, strongly suggest that VIRLAZA could be successfully used in the early phase for COVID-19 patients, to avoid the aggravation of the disease, which happens due to intense synthesis and release of pro- inflammatory cytokines by bronchial epithelial cells. In terms of possible immunological mechanism underlying such effects, the present study revealed that VIRLAZA induced synthesis and release of IL-1RA in BEAS-2B cells, which is a potent endogenous anti- inflammatory cytokine. In addition, following this initial pro-inflammatory response, an activation of lung fibroblasts is seemed in COVID-19 patients, which leads to lung fibrosis, compromise the lung function, the quality of life and the general health status of such patients for a long period. In this case, the present study demonstrated that VIRLAZA efficiently reduced lung fibroblasts (MRC-5 cells) activation, since VIRLAZA reduced the synthesis and release of IL-1beta (p<0.001), IL-6 (p<0.001) and TNF-alpha (p<0.001), which are classical cytokines used as biomarker of fibroblast activation. Importantly, the levels of IL-8 were not changed in MRC-5 after stimulation with infected blood of COVID-19 patients and also not changed after VIRLAZA stimulation. In this sense, is plausible to hypothesize that VIRLAZA, also could inhibit the lung fibrosis observed in more severe cases of COVID-19. Again, concerning the possible anti-inflammatory immunological mechanism involved in the effects ofVIRLAZA, its was able to induce the synthesis and release of the anti-inflammatory cytokine IL-1RA, which is the trigger anti-inflammatory cytokine suggesting deactivation of lung fibroblasts, induced by COVID-19. So, with theseconsistent results, we are able to prove a potent anti-inflammatory and anti-fibrotic effect of VIRLAZA in the context ofCOVID-19, open a new opportunity for early intervention in individuals with COVID-19. Thus, considering such results, a clinical trial using VIRLAZA for COVID-19 patients from different severities are urgently required.
The graphs presenting all the results are displayed bellow.
***p<0.001; **p<0.01 and *p<0.05. Co = control group (only the cells);
VR = VIRLAZA group (cells + VIRLAZA);
SarsCov2 = Infected group (cells incubated with blood of patient infected with COVID-19) and SarsCov2 + VR = Infected group treated with VIRLAZA.
We clarify that the levels of IL-10 (an immunomodulatory and sometimes anti-inflammatory cytokine) were also measured, but no differences among all groups were found.


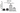
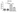
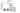


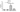




Development and research of the company "LibiPharm" - "LibiPharm" Israel.
© 2023 Calpton SIA. All rights reserved.
We accept




